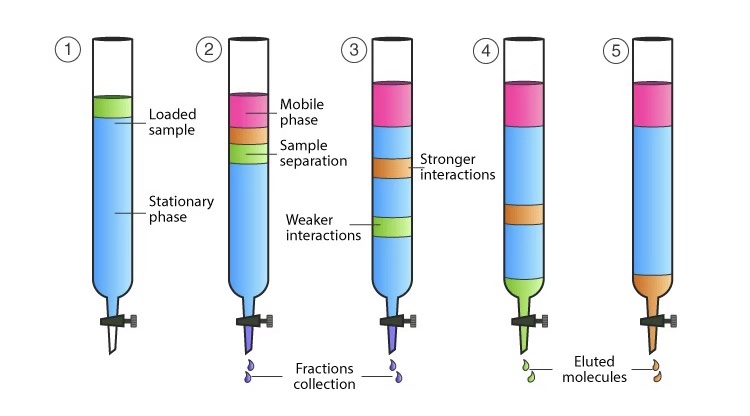
Chromatography
Chromatography is used to separate components in a mixture. In paper chromatography, a drop of a mixture is placed near the edge of the paper and dried. The paper is then placed into a developing chamber that holds the solvent, which flows along the sheet, carrying with it the compounds of the mixture at different rates depending on their solubility. The more soluble compounds will move further up the paper. After the paper is dried, the positions of the different compounds can be visualized.
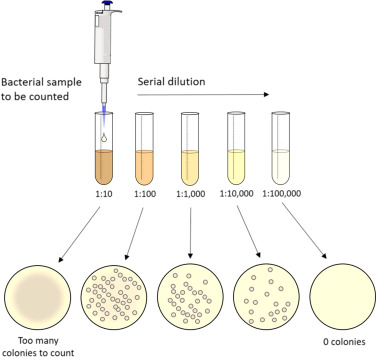
Dilution
Concentrations are defined as the amount of substance (called “solute”) per unit of solution. The typical unit for concentration is molarity (unit: M), which is the moles of a solute per liter of a solution. A dilution will take a concentrated solution and lower its molarity. This is highly useful in a lab setting since different experiments will require different concentrations of solutions so a stock (highly concentrated solution) can be diluted down according to what’s needed.
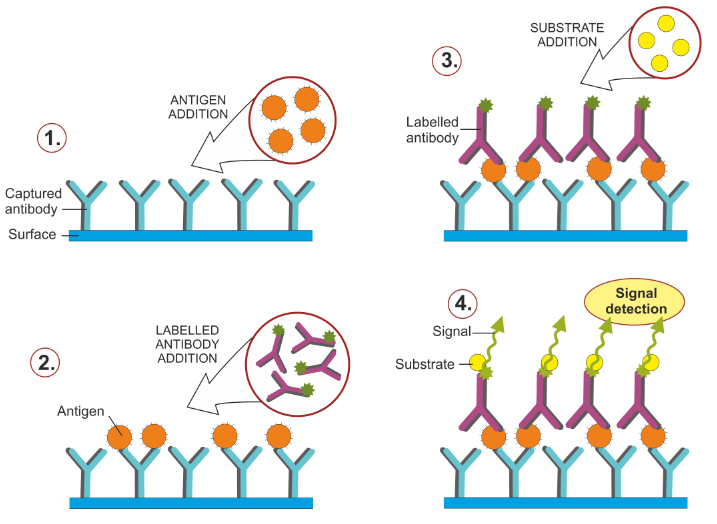
ELISA
ELISA (enzyme-linked immunosorbent assay) is used to identify and measure soluble molecules such peptides, proteins, antibodies, and hormones. The molecule that the experimenters are trying to target (antigen) is bound to an antibody which is connected to a reporter enzyme. The enzyme will produce some sort of reaction when the enzyme is bound such as releasing a color, which allows for proper identification of the target molecule.
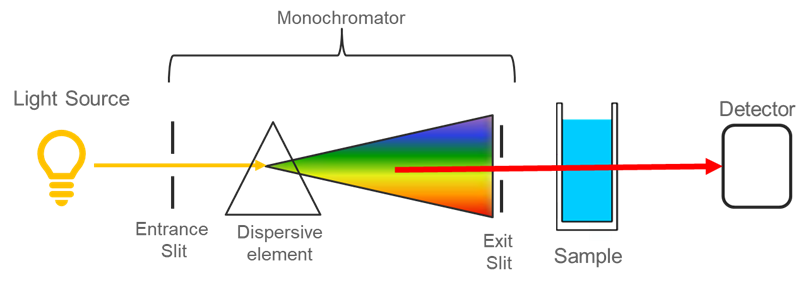
Spectroscopy
Measure of the wavelengths of light that are absorbed by different materials. All materials have a peak absorbance wavelength (λmax), which is the wavelength of light that is absorbed best by the material. In other words, if all wavelengths of light are shined at the material, the light with a wavelength near the λmax will be mostly absorbed while all other wavelengths of light will pass through (or be reflected by) the material. Detectors can measure the amount of light absorbed/transmitted.
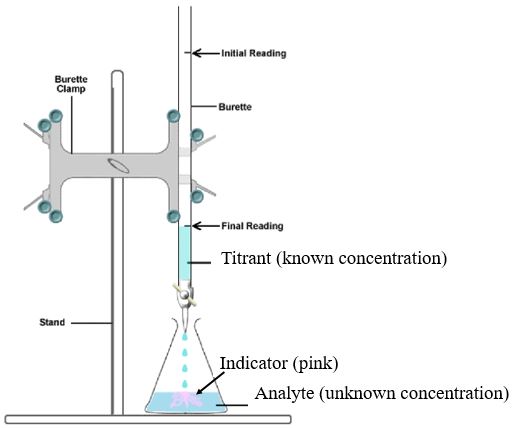
Titration
Titration is a laboratory technique used to determine the unknown concentration of acid (or base) in a solution. This is achieved by adding an indicator, which is a substance that rapidly changes color at a certain pH, and then slowly adding base (or acid), known as titrant, to neutralize the solution. Once the solution reaches a certain pH, the solution will change color due to the indicator. Based on the volume of titrant needed to reach this point, the initial concentration of the solution can be calculated.